Black Soldier Fly Antifreeze Proteins
The Problem
Foods, laboratory materials, and biomedical products are often frozen for long-term storage. However, ice recrystallization causes deterioration of these products, resulting in economic loss. Antifreeze proteins (AFPs) are used to minimize ice recrystallization.
The Solution
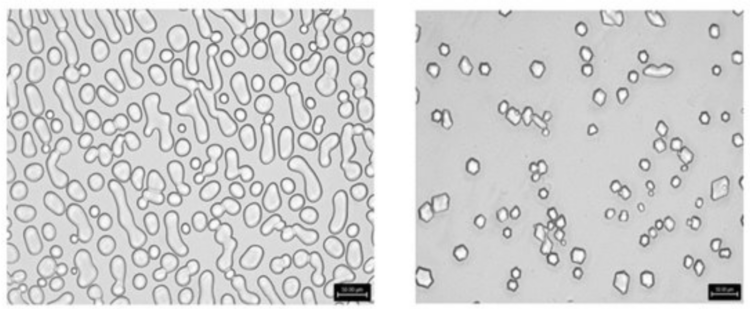
Researchers at the University of Tennessee have developed a technology that demonstrates the extraction and characterization of a black soldier fly larvae (BSFL) protein complex that is water soluble and has strong ice recrystallization inhibition (IRI) activity. Experiments showed that there was high IRI activity in the larvae specifically, while there was none in the adult flies.
Insect AFPs may be a better solution than other traditionally used AFPs, because:
- Synthetic AFPs are toxic and have limited applications
- Plant-based AFPs depend on fluctuating agricultural harvests
- Animal-based AFPs require additional processing of butchered livestock waste and are not suitable for health-conscious applications
Benefits
| Benefit |
|---|
| Lower production costs compared to animal AFP processing |
| Less land, water, and emissions than traditional livestock processing required to harvest nutritionally equivalent output |
| Insect AFPs can survive in colder temperature and bind to more ice crystals than commonly used fish AFPs |
| Recycle farming waste by using it as feed for the BSFL, who then can be harvested for their high-protein content |
More Information
- Tyler Newton
- Assistant Technology Manager
- 865-974-1882 | cnewto12@tennessee.edu
- UTRF Reference ID: 24143
- Patent Status:

Innovators
Dr. Toni Wang

Professor, Department of Food Science, College of Arts and Sciences, UT Knoxville
Dr. Wang received her PhD from Iowa State University in Food and Lipid Chemistry. Dr. Wang's research (with a total of ~$13 million funding) is focused on identifying novel and practical solutions for problems and challenges associated with processing, utilization, and functionalities of agricultural products intended for food, feed, biomaterials, and energy uses. She aims to use fundamental chemistry of lipids and proteins in new ways to improve food quality, functionality, safety, and health, and to reduce waste and environmental impact.
Read more about Dr. Toni Wang