Efficient Nanocatalyst for Hydrogen Gas Production from Water Splitting
The Problem
The efficiency and large-scale industrialization of the water splitting reaction is limited by the sluggish kinetics of the four electron-proton transfer process involved in oxygen evolution reaction (OER). Precious metal oxide electrocatalysts have been used to reduce the overpotential required for the OER due to their high catalytic activity, but their scarcity, high cost and instability present major drawbacks. Cerium oxides are a cheaper alternative but are not as efficient.
The Solution
Researchers at the University of Tennessee have synthesized a novel, compositionally complex, cerate-based fluorite and rare-earth (RE) cation nanocatalyst. These catalysts have been shown to display high catalytic activity and stability in an alkaline media for the OER and CO2 reduction reactions. These RE-cerium oxides have higher turnover frequency than existing cerium oxide catalysts, meaning more hydrogen gas product can be gained from the water splitting redox reactions. The RE-cerium oxides also reduce the overpotential energy required for the OER to progress, making the reaction more efficient.
Catalytic activity measured by turnover frequency (left) and overpotential (right)
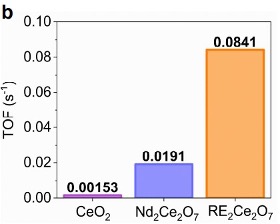
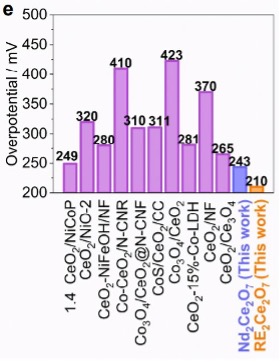
Benefits
| Benefit |
|---|
| Less expensive than precious metal catalysts due to increased abundance |
| Improved catalytic rate compared to existing cerium oxide catalysts |
| Cerium is mechanically stable and corrosion resistant in alkaline environments |
More Information
- Kusum Rathore, Ph.D.
- UTRF Vice President
- 865-974-1882 | krathore@tennessee.edu
- UTRF Reference ID: 23141
- Patent Status: Patent Pending

Innovators
Katharine Page

Assistant Professor, Department of Materials Science and Engineering, UT Knoxville
Dr. Page received her PhD from the University of California, Santa Barbara. Her research interests include new insights into complex functional materials (both bulk and nano) through advances in structural characterization, ferroelectric oxides, energy conversion materials, and nanoscale catalysts.
Read more about Katharine PageSreya Paladugu

Graduate Research Assistant, Department of Materials Science and Engineering, UT Knoxville
Dr. Paladugu received her PhD in Materials Science and Engineering from UT in 2023. Her PhD thesis was on Probing the Structure-Property Relationships of Metal Oxide Nanocatalysts.
Read more about Sreya Paladugu