LumenSecure: Evidence of IV Line Access Tampering
The Problem
For endocarditis patients who require intravenous drugs (IVs), the risk for patient-tampering with the peripherally inserted central catheter line (“PICC line”) to occur is quite high, which may lead to central-line associated bloodstream infection (CLABSI) of the patient. Such infections place a great cost on the hospital, which is often not reimbursed for such costs by insurance companies. Furthermore, since patients with CLABSI cannot be discharged to outpatient beds, hospitals are also unable to free up patient beds quickly and efficiently when such infections occur.
The Solution
Researchers at the University of Tennessee have developed a tamper-evident device which points out and discourages unauthorized tampering with the central line of IV systems. The device has a specially contoured enclosure that covers the lumen interface points in the central line and shows clear evidence of when the central line has been tampered with.
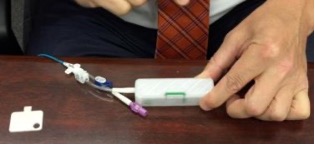
Benefits
| Benefit |
|---|
| Easy to use with minimal impact on nurse workflow. |
| Studies performed at the University of Tennessee Medical Center and Indiana’s Memorial Hospital and Health Care Center showed promising results: nurses surveyed indicated ease of use of the device and unambiguous tampering evidence. |
| Counts as sufficient documentation for CDC CLABSI rule, meaning the infection won’t count against the facility. |
More Information
- Kusum Rathore
- UTRF Vice President
- 865-974-1882 | krathore@tennessee.edu
- UTRF Reference ID: 16061
- Patent Status: 10,722,680
Innovators
Matthew Mench

Director, Electrochemical Energy Storage and Conversion Laboratory; Dean and Chancellor's Professor, Tickle College of Engineering
Dr. Mench received his Ph.D. in Mechanical Engineering from Pennsylvania State University in 2000. He serves as the Dean for the Tickle College of Engineering. Dr. Mench serves as the director for the Electrochemical Energy Storage and Conservation Laboratory at the College (EESC).
Read more about Matthew Mench