By Kimberly Hood
 It’s hard to find what you can’t see, and it’s difficult to diagnose what you can’t find. This is exactly the problem Solex LLC is trying to correct in the detection of amyloidosis. Every year 3,000 Americans are diagnosed with amyloidosis, but currently, there is no imaging technique available in the U.S. to detect the disease. Thus, there is no way for doctors to actually see the problem.
It’s hard to find what you can’t see, and it’s difficult to diagnose what you can’t find. This is exactly the problem Solex LLC is trying to correct in the detection of amyloidosis. Every year 3,000 Americans are diagnosed with amyloidosis, but currently, there is no imaging technique available in the U.S. to detect the disease. Thus, there is no way for doctors to actually see the problem.
What is Amyloidosis?
Amyloidosis is a disease that occurs when abnormal proteins begin to build up in internal organs. It may occur in conjunction with many common diseases including Type 2 diabetes, myeloma, rheumatoid arthritis and Alzheimer’s. The symptoms of amyloidosis vary widely based on which organs are affected, so diagnosis is often delayed. Once diagnosed, the median survival rate of amyloidosis is only four years. Currently the only way amyloidosis may be diagnosed in the U.S. is through a biopsy. However, even with a biopsy diagnosis, doctors have no way of knowing the extent of the protein build up or the number of organs affected.
The Solution
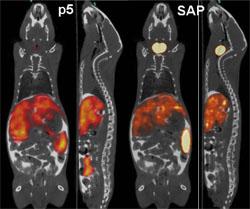 Researchers at the University of Tennessee Medical Center have developed a synthetic peptide radiotracer for use with Positron Emission Tomography/Computed Tomography (PET/CT) imaging devices that facilitates the non-invasive, whole-body visualization of the amyloid burden in patients. “PET imaging detects radioactive material,” Solex CEO, Jonathan Wall, Ph.D. said. “The peptide is a protein with a radioactive tag. It binds to the amyloid and gives an exact picture of where the amyloid burden is. It can be in the body for quite a while and generally no one realizes it until it has built up to the point of at least partial organ failure.” The earlier amyloidosis is detected, the sooner doctors can begin working to prevent additional organ damage by stabilizing the function of affected organs and treating the underlying condition(s) causing the amyloid build up.
Researchers at the University of Tennessee Medical Center have developed a synthetic peptide radiotracer for use with Positron Emission Tomography/Computed Tomography (PET/CT) imaging devices that facilitates the non-invasive, whole-body visualization of the amyloid burden in patients. “PET imaging detects radioactive material,” Solex CEO, Jonathan Wall, Ph.D. said. “The peptide is a protein with a radioactive tag. It binds to the amyloid and gives an exact picture of where the amyloid burden is. It can be in the body for quite a while and generally no one realizes it until it has built up to the point of at least partial organ failure.” The earlier amyloidosis is detected, the sooner doctors can begin working to prevent additional organ damage by stabilizing the function of affected organs and treating the underlying condition(s) causing the amyloid build up.
How Solex Began
While their team has been researching new imaging agents since 2008, Solex, the company based on the technology, has only been in existence for a few months. “Solex was born out of a homework assignment and the need for clinical trials,” Wall said. “Emily Martin, my grad student, was taking Fred Tompkin’s course and one of her projects was to come back to him with something from the lab that they could turn into an entrepreneurial project.” It was this homework assignment, coupled with the need to secure Small Business Innovation Research (SBIR) funding in order to proceed with clinical trials in humans, which led to the formation of Solex. The inventors negotiated the right to license the intellectual property from the University of Tennessee Research Foundation and the deal was finalized in December 2011, making it the first start-up company formed out of the University of Tennessee Medical Center.
Looking to the Future
 Wall was quick to point out that forming the company was not their biggest obstacle, but instead, the difficult part was securing the funds necessary to advance the technology through trials required for FDA approval. “The major hurdle is not to start the company,” Wall said. “It’s to not go bankrupt, lose your house or your kidney early on.” Now that the company is established, their biggest concern is lining up investors and applying for SBIR grants to fund further testing. Solex has applied for SBIR Phase I funding. If everything goes according to plan, they will apply for a SBIR Phase II grant in April which would fund clinical trials in humans. After funding is acquired, Wall says that human clinical trials can be under way in as little as 12 months. Meanwhile, Solex is working toward manufacturing staining kits to aid clinical pathology laboratories in detecting amyloid in samples. They also are conducting additional in-house trials in mice. Solex developers from the UT Graduate School of Medicine include Jonathan Wall, Ph.D., Stephen Kennel, Ph.D., Emily Martin, B.S., Tina Richey, M.S., and Alan Stuckey, B.A., CNMT. Visit their website
Wall was quick to point out that forming the company was not their biggest obstacle, but instead, the difficult part was securing the funds necessary to advance the technology through trials required for FDA approval. “The major hurdle is not to start the company,” Wall said. “It’s to not go bankrupt, lose your house or your kidney early on.” Now that the company is established, their biggest concern is lining up investors and applying for SBIR grants to fund further testing. Solex has applied for SBIR Phase I funding. If everything goes according to plan, they will apply for a SBIR Phase II grant in April which would fund clinical trials in humans. After funding is acquired, Wall says that human clinical trials can be under way in as little as 12 months. Meanwhile, Solex is working toward manufacturing staining kits to aid clinical pathology laboratories in detecting amyloid in samples. They also are conducting additional in-house trials in mice. Solex developers from the UT Graduate School of Medicine include Jonathan Wall, Ph.D., Stephen Kennel, Ph.D., Emily Martin, B.S., Tina Richey, M.S., and Alan Stuckey, B.A., CNMT. Visit their website