Stable, High Voltage LiMn1.5Ni0.5O4 (LMNO) Cathode for Lithium-ion Batteries
The Problem
Cathode material selection has traditionally dominated the cost and energy-density limits of Lithium-ion batteries. Spinel oxide cathode chemistries such as LMNO demonstrate high-energy density and high operating voltage but have been hindered by rapid capacity decay during cycling because of the unstable structure of the manganese and nickel elements along with Jahn-Teller distortion effects.
The Solution
Researchers at the University of Tennessee have developed a new LMNO cathode and optimized synthesis method which minimizes capacity fade during cycling, enhances the stoichiometric ratio for LMNO production, and retains high phase purity. This technology’s chemical synthesis method features a toxic-free precursor for greater stability, nanoparticle surface modifications, and a network of carbon nanotubes to facilitate fast lithium-ion transport. Production of LMNO cathode in this manner will limit instability in cycling while realizing the advantages of high energy density and operating voltage of LMNO. Applications include EVs, e-VTOL aircraft, grid energy storage, and other high-Voltage Li-battery applications.


Benefits
| Benefit |
|---|
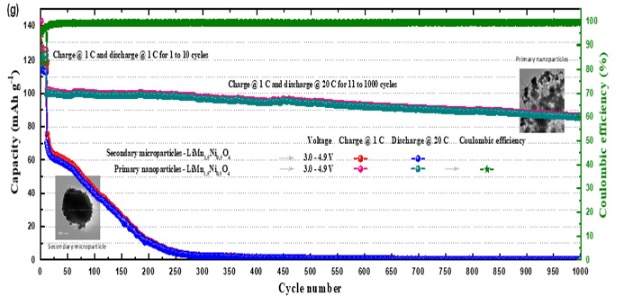
| Extended Performance - primary LMNO nanoparticles exhibit very small capacity dropoff after 1000 cycles (only 14 mAh g-1). |
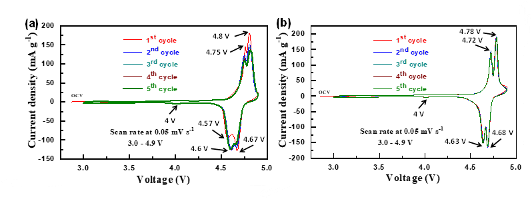
| High Density - energy densities as high as 650 W-h/kg, at a discharge plateau voltage of 4.7 V. |
| Scalable – facile chemical synthesis method. |
| Environmentally Safe -Cobalt-free cathode and non-toxic precursor. |
| Planned improvement to primary nanoparticle and secondary microparticle sizes for faster Li+ ion transport and lower diffusion length. |
More Information
- Gregory Sechrist
- Technology Manager
- 865-974-1882 | gsechris@tennessee.edu
- UTRF Reference ID: 23147
- Patent Status: Patent Pending

Innovators
Manikandan Palanisamy

Research Assistant Professor, Department of Mechanical, Aerospace, and Biomedical Engineering (MABE), Tickle College of Engineering, UT Knoxville
Dr. Palanisamy received his Ph.D. from the CSIR -Central Electrochemical Research Institute in 2015 in India. He is a research assistant professor for the MABE Department at UT. His research interests include Lithium-ion batteries, Solid-State Chemistry, and Crystal Structure studies.
Read more about Manikandan PalanisamyMatthew Mench

Director, Electrochemical Energy Storage and Conversion Laboratory; Dean and Chancellor's Professor, Tickle College of Engineering
Dr. Mench received his Ph.D. in Mechanical Engineering from Pennsylvania State University in 2000. He serves as the Dean for the Tickle College of Engineering. Dr. Mench serves as the director for the Electrochemical Energy Storage and Conservation Laboratory at the College (EESC).
Read more about Matthew MenchDoug Aaron

Research Assistant Professor, Electrochemical Energy Storage and Conversion Laboratory; Assistant Department Head, Department of Mechanical, Aerospace, and Biomedical Engineering
Dr. Aaron received his Ph.D. from Georgia Institute of Technology in 2010, and serves as the assistant department head for MABE at UT and serves on the research staff of the EESC laboratory.
Read more about Doug Aaron